Considerations for Treatment in Cold-Climate Regions
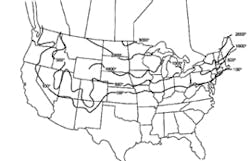
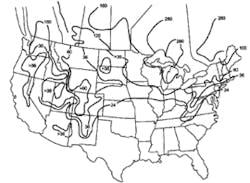
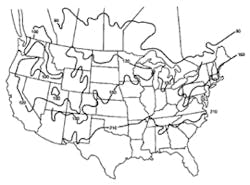
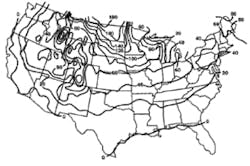
Stormwater treatment in cold-climate regions involves considerable additional difficulties than with similar treatment systems in semi-arid and wet climate regions. Cold regions can be defined by Figures 1 through 3. Figure 1 presents air freezing index values, which are the cumulative degree-days below 32°F. It has been suggested that ice cover will occur on lakes in regions with values greater than 100. Figures 2 and 3 present annual snowfall depths and length of freeze free period. Figures 4 through 6 are relevant to the question of how deep treatment systems must be placed subsurface to avoid freezing—for examples, fine-media filters and wet vaults. The information provides perspective. More definitive data available by state or province should be consulted. This article present’s a general review of the author’s experience.
Issues
Table 1 lists issues facing treatment in climatic regions where substantial freezing occurs. The table expands on summaries provided by others. Caution is warranted with many of the suggestions given lack of field experience. Most of the adjustments in Table 1 are discussed more fully elsewhere (Minton 2011). These discussions should be consulted, as some adjustments may conflict, be inappropriate, or be of inconsequential value in certain situations. Some issues may be overstated depending on the particular locale.
Snowmelt has a unique dynamic. Pollutants accumulate in the snow over the winter. Accumulation is variable, affected by traffic volume, snow age, prevailing winds, curbs, the amounts of deicing salt or traction sand used in the drainage area, and the characteristics of the drainage system.
Stormwater Quality
Concentrations of pollutants in snowmelt from freeways are commonly 1.5 to three times those in normal runoff, varying with the pollutant and the particular freeway. Snowmelt can be very acidic, with pH values below five. The pH may increase with increasing traffic. Of particular concern is the use of deicing salts, in particular sodium chloride. Heavy metals in the presence of chloride tend toward the dissolved state, but low temperatures are a major factor as well. It appears that low temperatures have as much an effect as chloride on the solubilization of metals. Sediment concentrations may be much greater in the spring melt due to the use of traction sand and/or the extended period of atmospheric deposition on snow piles. The spring melt may represent a disproportional fraction of the total annual loading of many pollutants. Also of concern is cyanide in deicing salts, which serves as an anti-caking amendment. Total suspended solids (TSS) are also typically higher in cold-climate regions than in temperate and semi-arid regions, on the order of 300 to 500 milligrams per liter (mg/L),
versus 50 to 150 mg/L.
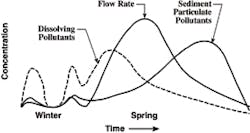
The idealized rendition of the relative concentrations of pollutants in the spring melt is shown in Figure 7. The figure, of course, assumes one long melt, which is not always the case. But it has been proven that the concentrations of dissolved pollutants are greater during the early part of the melt, and that the concentration of TSS is higher during the later part of the event. The timing of the TSS concentration relates to fine sediments being blown onto the top of the snow pile from traffic. Hence, if there are several snow events with long inter-event periods of dryness, the idealization shown in Figure 7 will not be realized. The graphic is also reflective of snow piles next to busy roads. The relative and absolute increase in TSS will be much less in residential areas. As most of the TSS is on the surface of the basin, it does not appear at significant concentrations until the snow pile is considerably lower, as indicated in Figure 7.
Ice Formation
It has been suggested that depth be added to wet ponds to account for space taken by ice and snow. The adjustment is to maintain sufficient liquid volume for acceptable performance during winter and spring melts.
Two options are considered:
- Increase water depth beneath ice.
- Drain the wet pool prior to the freeze period and operate as an extended detention basin during winter and spring melts.
Increasing water depth to accommodate ice may conflict with water depth requirements in the summer. Experience with scouring suggests a minimum winter depth of perhaps 6 feet (2 meters). However, 6 feet is the likely maximum desirable depth to avoid permanent stratification during the summer. Anaerobiosis at the bottom of the basin occurs if either thermal or saline stratification exists, with attendant performance implications.
Option 1 is perhaps viable if winter ice is relatively thin, a foot (0.3 meter) or less, but not with an extreme thickness of 3 feet (1 meter).
Option 2 may be more appropriate for these areas. Alternatively, the outlet structure can be designed to allow operation at two different depths: a liquid depth of 6 feet during the summer, and 6 feet plus the thickness of the ice during the winter. Ice thickness can be estimated with Equation 1. Local experience may suffice.
Di = a(Fd)0.5
Where:
Di = ice thickness in millimeters
a = coefficient of ice growth
Fd = the sum of freezing degree-days
Values for the coefficient are 27 for windy lakes without snow and 17 to 24 with snow. A value of 15 has been found valid for ponds in southern Ontario.
An option to prevent ice formation is bottom aeration, likely employed only in exceptional situations. Mixing by aeration or some other means prevents stratification.
Basins
Wet ponds and vaults, as well as wetlands, may be preferred by default given inadequacies and risks of freezing attendant to other systems such as swales and fine-media filters. Although the performance of basins in cold climates may degrade in comparison to their counterparts in temperate and semi-tropical regions, their performance may be better than the alternatives available for cold climates.
Stratification is the occurrence of two layers of different density in a wet basin. The impact on hydraulic efficiency is a dead zone (the bottom) and enhanced short-circuiting. It occurs two ways: temperature and salinity. Saline stratification has been observed in small bays, small lakes, and stormwater ponds and vaults. Permanent stratification throughout the summer can occur depending on the concentration of chloride, depth of the basin, and strength of winds. However, the effect on performance of chloride stratification in cold climates may be little different than the effect of thermal stratification in temperate and semi-tropical regions.
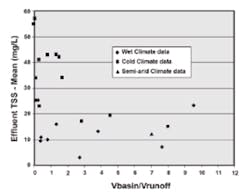
High concentrations of chloride in the melt water cause saline stratification during the winter and spring melts. Metals in the soil under anaerobic conditions solubilize, are released into the water, and may be lost in the spring melt. Zinc desorbs from the surfaces of cattails in the winter, but this also occurs in other climate areas. Concentrations of TSS tend to be greater in the effluents from basins in cold regions (Figure 8). Settling rates will be lower. This may be due as much to higher influent concentrations (deicing sand) as to cold temperatures and stratification. Seasonal comparison of the removal efficiencies of a pond showed that removal of cadmium and copper was about the same for summer and winter-spring, but removal of lead, zinc, and TSS dropped from the summer to the winter.
That metals redissolve when the ponds become anaerobic is counter to what is known about metal removal and loss. We know that the two primary mechanisms of dissolved metals removal are precipitation to sulfide and sorption to organic matter. The precipitate can exist only in an aerobic situation. The metal-sulfide precipitate redissolves in the lower depths of the basin become anaerobic. Thus, the release cannot be from the metal-sulfide complex. It is unlikely that the chloride dissolves the metal-sulfide complex. While not known, it is very unlikely that the metals desorb from the organic matter. The most plausible mechanism is not the release of metals from the bottom. It is more likely that higher percentage of the metals in the snowmelts remain in the dissolved state due to low temperature and chloride.
Depending on several factors, in particular wet pool depth, stratification may remain through the summer, creating essentially a permanent dead zone at the bottom of wet ponds and vaults. This decreases hydraulic efficiency, and may adversely affect the removal of dissolved pollutants. Higher-than-background chloride concentrations have been found to persist in the discharge throughout the summer but perhaps not at deleterious levels. The larger basin recommended in this article will compensate for lower settling velocities during the spring melt.
Equation 2, derived from estuaries, has been offered to evaluate whether saline stratification will persist in the summer. Note that the velocity of the wind is not included. Also note that Q is the average flow rate. It can be base flow or the average flow rate during a storm. Regardless, the results are therefore conservative.
If the Froude Number is much less than 0.3, the potential for stratification is strong; if F is near 0.3, intermittent or weak stratification is likely; if F is much greater than 0.3, the wet pool remains well mixed. These values are based on limited laboratory studies in flumes.
(Equation 2)
F = ______Q_________
DW(((Ïs – Ï)/Ï)gD)0.5
Where:
Ï = density of stormwater
Ïs = density of the salt layer
F = Froude Number
Q = average basin discharge rate
D = mean water depth
W = basin width
g = gravity constant
The results from Equation 2 and a similar equation for thermal stratification support the view that salinity stratification is more stable than thermal stratification.
High concentrations of chloride cause toxicity to wetland plants, altering the diversity of plant life. The formation of ice results in low or zero dissolved oxygen concentration at the bottom of the basin, and possibly throughout the vertical profile of the basin. Metals in the soil, under anaerobic conditions, solubilize, are released into the overlying water, and may be lost in the spring melt. Zinc desorbs from cattails in the winter, but this also occurs in temperate regions. Concentrations of TSS tend to be greater in the effluents from basins in cold regions. (See Figure 8.) However, this may be due as much to higher influent concentrations (deicing sand) as to cold temperatures and salinity (denser water).
Despite the higher effluent concentrations of TSS in cold climates, the generally accepted removal goal of 80% is reached. The influent concentrations to the cold basins shown in Figure 8 were generally in the range of 200 to 500 mg/L, whereas the influent to basins in the temperate climate regions were on the order of 50 to 100 mg/L. Perhaps the performance goal should be 90% for cold climate regions. As indicated in Figure 8, a better effluent quality can be reached with a larger basin, relative to that needed in temperate regions.
It has been found that the TSS in sediment and chloride laden melts settles at about half the rate experienced during less-contaminated summer melts. This was observed in small vaults. Differential settling rates may not be a factor in large basins.
It is commonly believed that lower performance occurs during melts, but the field data are inconsistent. While this is a legitimate concern, field studies suggest that lower performance may be due more to bottom scour at the entrance than degraded removal processes. However, the studies of scour are inconsistent, likely due to differences in the geometry of the entrance. A deep entrance or a hardened entrance can prevent scour.
The strength of stratification and its effect on processes within the wet pool are affected by whether the ice remains throughout the winter. Intermittent melting of the ice may result in the re-oxygenation of the basin, particularly with winds, and the re-precipitation or sorption of metals previously dissolved. Thus, the impact of ice cover depends upon the latitude and nearness to large bodies of water that tend to moderate atmospheric temperatures.
Biological activity is slower in the winter. However, with little flow, the issue is not particularly relevant except during the spring melt. There are two biological activities of importance. One is the nitrogen processes. Nitrification (conversion of ammonia to nitrate) and denitrification (nitrate to nitrogen gas) slow significantly below 15°C (59°F) and essentially cease below 5°C (41°F). However, ammonia concentrations in stormwater are not at concentrations sufficient to be toxic to receiving water biota. Ammonia is relevant only if the discharge is to nitrogen-limited streams. The other biological activity is the formation of sulfides, which, as with nitrogen processes, are performed by specialized bacteria. Sulfide is important for the sequestering of dissolved metals, drawing the metals into the soil. While plant activity reduces significantly at low temperatures, plants were found to be beneficial with respect to performance at water temperatures as low as 4°C (39.2°F). These tests were conducted with small pilot units.
A reasonable question is whether poorer performance during winter or spring melts is of concern. At very low water temperature, plants as well as fish and other aquatic organisms are dormant or absent. The metabolism of fish in cold receiving waters is low and therefore less affected by toxic pollutants.
Swales and Strips
The issue with flow-through swales is dormancy of the grass during the spring melt. Several spring storms may occur before the grass is fully functional. As a consequence, grass swales may not be an appropriate as the main treatment device and are of use only where significant infiltration occurs are of particular benefit for spring melt. If present, significant infiltration helps performance. However, it has been found that strips bordering agricultural fields are effective in the winter, perhaps due to the grass being taller than in urban swales. I have taught a short course in cold-climate regions in the United States and Canada, and none of the attendees when asked has indicated any concern about swales performing during the spring melt. It is possible that even dormant grass reduces the velocity of the stormwater sufficiently to allow TSS and attached pollutants to settle. Regardless, the question has not been researched and therefore remains a concern.
Filters and Infiltration
These two types of treatment systems respond similarly and therefore are discussed together. Fine media (and by extension, course media), if not saturated, will not freeze, but the hydraulic conductivity will be reduced. Studies of porous pavement, and experiments by the author, show that wetted sand will freeze but that gaps remain between the sand particles. With each successive freeze, a new layer of frozen water forms on the surface of the sand. A study of porous pavement found that at least 11 successive freeze/thaw events occurred before the hydraulic conductivity approached that of the design value used in the sizing of sand filters. Hence, sand filters should operate should operate well throughout the year. The surface of the filter perhaps should be cleaned before the winter season as fine material collected by the filter on its surface will freeze. However, this layer is very thin. As such, it would be expected that the frozen layer will be easily broken up if not melted by the incoming water. Organic media is most susceptible to freezing as it retains more moisture. As pellets or in a similar form, the organic media will lose its structural integrity.
If a significant percentage of the solids are fine particles, or if it is saturated, the soil is likely to freeze solid. However, infiltration basins are generally placed in coarse soils with less tendency to freeze as a solid. As with sand filters, soils do not freeze completely in all cases. Generally, the coarser the soil, the less extensive the freezing; the soil acts as a coarse media filter. As with filters, the only situation in which the soil totally freezes is if it is saturated. If, as with sand, freezing occurs only at the surface of the soil particle, the hydraulic conductivity may be sufficient for significant infiltration during the spring melt. However, the question has not been tested.
As with filters, porous pavement has not been found to freeze in cold regions. Porous pavement is used in the cold regions of Canada and northern Europe with no issue of freezing. Freezing is prevented by the relative warmth of the underlying ground. Porous pavement was found to freeze on an airfield. The difference between the results is likely due to the lower traffic on runways. Passing vehicles cause pressure on the ice, which results in thawing. There are fewer vehicles on a runway on a daily basis than on roads.
Strategies
Many alternative strategies are presented in Table 1, related to each specific problem. Some these strategies are discussed below.
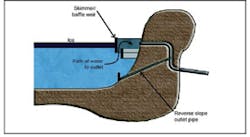
Pipes are placed below the frost line. Freezing of the influent pipe may occur at the discharge point if the discharge is at the surface of the basin. This is avoided by placing the discharge point at the bottom of the basin. But this problem has not been observed. The inlet area is hardened to prevent scour. Freezing of the outlet is avoided through the use of a baffle and subsurface discharge as shown in Figure 9.
One strategy for wet basins is to have melts pass over the top of the ice, providing treatment by extended detention. An alternative strategy is to bypass the main treatment unit during the freeze period and spring melt. Only the pretreatment settling unit provides treatment. The objective is to keep chloride-laden stormwater from entering the main treatment unit: wetland, filter, or infiltration basin.
Bypassing the main treatment unit protects this unit from damage, whether structural or biotic. It avoids loss of previously removed pollutants by bottom scour or by solubilization. Using this concept, the pretreatment unit would be sized to effectively treat intermittent winter melts and the spring melt. It also would be sized and configured to deal effectively with ice. Lower performance might be accepted when considering the total annual performance goal inasmuch as the environmental effect of discharges on the receiving water is likely less during periods of cold-water temperatures.
Examples of pretreatment units are forebays in ponds and wetlands, the pond in pond/wetland combinations, and a vault or small basin preceding a sand filter or infiltration unit. The pretreatment unit would have sufficient depth to avoid bottom scour, or alternatively may include mixing or bottom aeration operated at selected periods to avoid freezing as well as saline stratification. The unit may be cleaned after the spring melt to meet normal annual maintenance needs, but providing the ancillary benefit of removing the saline water, dissolved pollutants, and sediments that might desorb metals in the following winter. As the main treatment unit is used only during late spring through early fall, its maintenance cycle will be lengthened.
Another strategy is the use of subsurface wetlands, also called gravel wetlands. The outlet is placed 6 inches (15 centimeters) or so below the surface of the gravel, inhibiting freezing. This configuration also prevents the presence of mosquitoes.
An alternative strategy for extended detention basins is to place coarse media on the bottom with an underdrain system. Melts enter below the ice and pass through what is a coarse media filter. Wet basins could be drained and operated as extended detention basins during the winter.
As noted previously, permanent saline stratification has been observed in deeper areas of wet ponds, Stratification reduces hydraulic efficiency and removal of dissolved metals and phosphorus by bottom soils. Avoiding depths greater than 6 feet (2 meters) may avoid permanent stratification, according to limited field studies. However, this minimum depth conflicts with the need to have a similar unfrozen depth to provide vertical space for ice. Having a winter water depth of 6 feet plus the thickness of the ice results in a summer water depth in excess of 6 feet, unless the outlet structure is designed to allow operation at different water depths. This is less an issue, of course, the thinner the design ice cover.
It could be argued that intermittent winter melts should bypass the treatment system. Their volumes are generally small compared to the spring melt and summer events and may be more heavily laden with chloride. Bypassing the more highly concentrated small flows would minimize the potential for saline stratification and toxicity to wetland plants in the spring and summer. The potential deleterious effects of bypass may be less than the effect of storing the melt only to have it released in the spring when the effects may be more deleterious to biota in the receiving water. This alternative strategy may be preferable to using the main treatment unit during the winter.
What should be considered in the formulation of a strategy is the chloride (and pollutant) loading of each hydrologic period described below. Bypassing small melt volumes of heavily laden chloride waters may provide a better overall environmental protection strategy. Treating the winter melts with only the pretreatment unit may be the appropriate intermediate solution. It is worthy to note that absent the treatment system, high chloride concentrations would enter receiving waters anyway, without the benefit of treatment or dilution. This perspective should be kept in view when developing a treatment strategy.
As for the accumulation of snow, sufficient freeboard is provided, the depth dependent on the expected snowfall during the winter, at, say, a 10-year return frequency.
Four Flow Types
Sizing methods should consider four flow event types: winter melts, spring melt, rain-on-snow events, and rain-generated runoff events during the remainder of the year. Rain-on-snow events will have the greatest volume and likely flow rate. Whether this flow type should be the basis for sizing depends on frequency. It is recommended this question be considered in coastal areas and the Great Lakes area, where the annual snowfall exceeds about 3 feet (1 meter).
Of the remaining three flow types, winter melts have the smallest volume and flow rate compared to summer
storms (Figure 7). Comparing the remaining two, the spring melt generally has the greater volume but lower peak flow rate. Hence, sizing volume-based devices such as basins may be related to the spring melt, whereas the statistics of summer/fall events should control rate-based systems such as swales and small manufactured vaults and filters.
In regions with accumulated snowfall in excess of about 12 inches (33 centimeters), the volume of the spring melt will be greater than the volume of the design event based on runoff depth. While the volume of the spring melt is for many areas greater than rain events of statistical interest, runoff occurs over several days rather than a few hours. It therefore does not necessarily follow that the volume of volume-based systems should be increased in response to the greater volume of the spring melt.
Rather, the sizing method should be based on the desired performance, recognizing the possibility of slower settling velocities in colder water as previously mentioned. Also, sediment in the spring melt is generally finer than that of rain events unless traction sand dominates. However, research indicates that clays flocculate more quickly at very low temperatures, increasing settling velocities above that otherwise expected.
It is likely that the size of a wet basin can be based on the statistics of rain-generated runoff events occurring in the summer/fall period. Extended detention basins may be another matter. It is likely that these basins should be sized to fully treat the spring melt to ensure meeting the volume performance goal.
Methods for estimating snowmelt volume, and consideration for rain-on-snow events, are available. Snow-melt algorithms for simulation models have been reviewed. Reviews of the characteristics of snowmelt, effects on treatment systems, and appropriate design criteria are available, as well as guidelines for snow storage areas.
Reference
Minton, G. R. 2011. Stormwater Treatment: Biological, Chemical, and Engineering Principles. RPA Press.